
Sequencing of the genome of the native wine yeast Issatchenkia terricola and searching of enzymes of potential oenological application
Non-Saccharomyces yeasts are of interest in the wine industry because they produce and secrete enzymes that can lead to the release of desirable volatile compounds during winemaking, contributing to their varietal aromas. In this sense, the exploitation of native strains is essential to provide authenticity to local wines by imparting, among other desirable characteristics, regional character. The group of Dr. Paula González at the Faculty of Chemistry, UdelaR, isolated a non-Saccharomyces yeast, Issatchenkia terricola, which expresses a β-glucosidase that stands out for its stability under oenological conditions and for the aromatic characteristics it provides to young wines. In order to promote the exploitation of this native yeast as a source of glycosidases and other enzymes of potential oenological interest, we carried out in collaboration with Dr. González’s group the sequencing and annotation of the genome of I. terricola, followed by the search for enzymes of biotechnological interest. Focusing on the identified β-glucosidases, we are carrying out the heterologous expression and characterization of some of these enzymes, with a view to their biotechnological application.

Characterization of possible nucleoside and glucose transporters in the parasitic cestodes Hymenolepis microstoma and Echinococcus multilocularis
The tegument is a key tissue for the anchorage, protection and nutrition of the cestodes, among which are included several parasitic species of man and domestic animals. Dr. Uriel Koziol’s group has approached the study of this tissue, aiming to identify possible therapeutic targets for the design of new and better treatments against these pathogens infections. In H. microstoma and E. multilocularis, glucose and nucleoside transporters have been identified as good candidates, from transcripts obtained from RNAseq data. We have recently established a collaboration with Dr. Koziol’s group in order to functionally characterize these transporters, through their heterologous expression in Saccharomyces cerevisiae and A. nidulans.

Regulation of cold response genes in a Pseudomonas strain of Antarctic origin
Faced with a sudden drop in temperature, many bacteria stop their growth and synthesize a set of proteins that allow them to adapt to new conditions. Collaborating with the group of Dr. Susana Castro-Sowinski, we seek to contribute to the knowledge of the molecular mechanisms involved in adaptation to cold in psychrophilic bacteria, with an emphasis on their gene regulation.
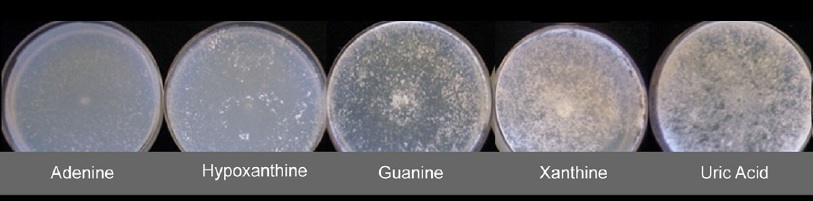
Structure-function relationships of basidiomycotas purine transporters
The group led by Dr. Gianna Ceccheto (Microbiology, Faculty of Sciences) is studying the structure-function relationship of new purine transporters identified in Basidiomycota (Phanerochaete chrysosporium¸ model organism, and Punctularia atropurpurascens, isolated from our region), through its heterologous expression in A. nidulans. Based on our experience in the study of the structure-function relationship of UreA, we have stablished a collaboration to assist them in this matter. This will contribute to the knowledge of purine uptake systems of wood degrading Basidiomycota fungi and thus advance towards the understanding of the response mechanisms to nitrogen limitations present in these organisms of biotechnological importance.

Recombinant expression of proteases in the genus Aspergillus
A collaboration has recently been established with Dr. Juan José Marizcurrena (Biochemistry Section – Faculty of Sciences) for the recombinant expression of proteases of biotechnological interest in the genus Aspergillus. As a first approach, the recombinant production of bovine chymosin in Aspergillus nidulans will be attempted. Chymosin is an aspartic protease that selectively cleaves k-casein from milk, inducing its coagulation, and is used in the dairy industry for cheese production. We are currently working on the heterologous production of this protein by optimizing its coding sequence (taking into account A. nidulans´ codon usage). Also its production performance will be analyzed. We also propose to use Aspergillus niger, a microorganism widely used in industry, as a recombinant expression platform.